Australian Scientists Turns “Forever Chemicals” into Harmless Fluoride: A Promising Photocatalytic Breakthrough
In the fight against persistent environmental pollutants, a new development offers a ray of hope. Australian researcher Dr. Cameron James Shearer et al. from Univesity of Adeladie, have unveiled a process that uses sunlight-activated photocatalysis to decompose PFAS (“forever chemicals”)—one of the most stubborn and pervasive classes of contaminants—into benign fluoride ions, marking a potential turning point for water treatment and environmental remediation.
NUTRIENT RECOVERY
Janani
8/25/20252 min read
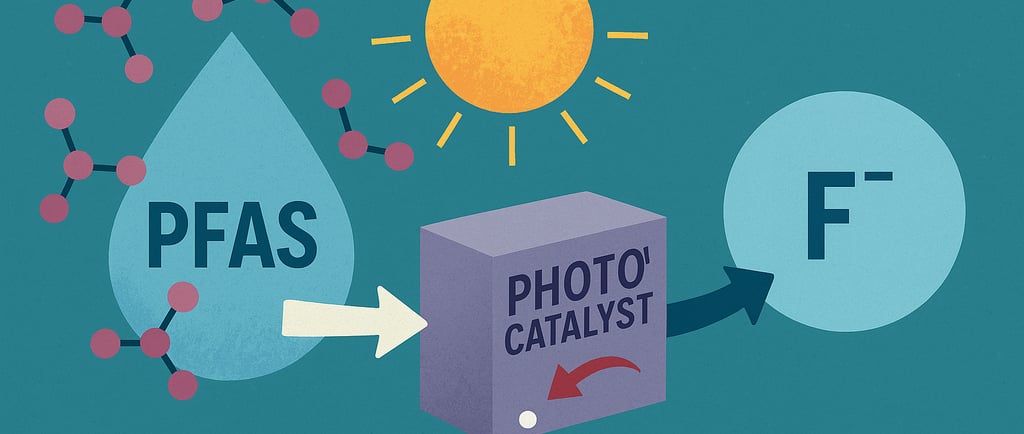
In the fight against persistent environmental pollutants, a new development offers a ray of hope. Australian researcher Dr. Cameron James Shearer et al. from Univesity of Adeladie, have unveiled a process that uses sunlight-activated photocatalysis to decompose PFAS (“forever chemicals”)—one of the most stubborn and pervasive classes of contaminants—into benign fluoride ions, marking a potential turning point for water treatment and environmental remediation.
Why PFAS Are Notorious—and Why It Matters
Per- and polyfluoroalkyl substances (PFAS) are synthetic compounds widely used in non-stick cookware, cosmetics, fire-fighting foams, waterproof textiles, and much more. They’re notoriously persistent due to their incredibly strong carbon–fluorine bonds—considered one of the toughest in organic chemistry—and accumulate in soils, water, and even human bodies . Alarmingly, nearly 98% of Americans now have detectable PFAS levels in their blood . These “forever chemicals” have been linked to a host of health issues, such as autoimmune diseases, developmental and fertility problems, and various cancers .
The Photocatalytic Innovation: Cadmium Indium Sulfide + Light
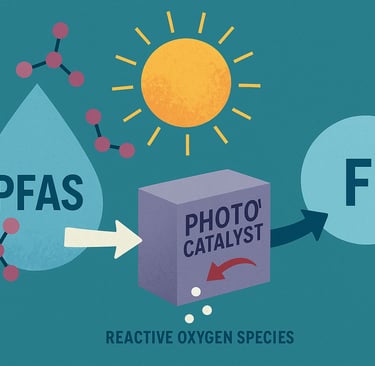
The breakthrough, published in Small, involves the use of cadmium indium sulfide—a visible-light–activated photocatalyst. When exposed to light, this material generates reactive oxygen species (free radicals) that can effectively cleave the resilient carbon–fluorine bonds in common PFAS compounds like PFOS (perfluorooctane sulfonate). Under optimized laboratory conditions, the process achieved up to 99% degradation of PFOS, yielding innocuous byproducts, primarily fluoride ions and potentially useful carbon remnants .
Toward Scalable PFAS Treatment
Lead researcher Cameron Shearer from the University of Adelaide emphasizes the method’s scalability potential. The envisioned model involves a two-phase system: first capturing and concentrating PFAS from contaminated water, then applying sunlight and photocatalyst treatment to degrade the pollutants. The end result is cleaner water—minus the PFAS load—all while potentially recovering fluoride for applications like toothpaste or fertilizer additives .
Implications & Synergies
Environmentally Safe: This method avoids harmful byproducts often associated with incineration or chemical oxidation, offering a cleaner alternative.
Energy Efficiency: As a light-driven process, it could be adapted for solar-powered systems—especially promising for decentralized or off-grid applications.
Reusability: The fluoride generated can be repurposed, supporting circular economy principles.
Integration Potential: Complementary to other PFAS-breakdown strategies, such as the heat–alkaline degradation method using sodium hydroxide in dimethyl sulfoxide .
Closing Thoughts
This sunlight-driven photocatalytic approach represents a significant advance in addressing one of modern environmental chemistry’s most persistent challenges. As the field moves toward scalable, cost-effective solutions, such innovations could redefine how we treat PFAS-contaminated water—bringing us closer to detoxified ecosystems and healthier communities.
